Page 30 - Summer 2010
P. 30
phometry and cortical thickness (Hyde et al., 2006; Hyde et al., 2007; Mandell et al., 2007), two measures of structure of grey matter in the brain, found that tone-deaf individuals possess differences in the superior temporal and inferior frontal areas. These differences were reported as being spe- cific to the right hemisphere by Hyde et al.. (2007), but were found to be involving more left fronto-temporal regions by Mandell et al.. (2007). The hemispheric differences between the effects observed may arise from different samples of sub- jects, different data analysis techniques in analyzing structur- al neuroimaging data, and different thresholding techniques employed in the published studies. In particular, left-hemi- sphere differences were observed using the dependent vari- able of grey matter signal, whereas right-hemisphere differ- ences were observed using the dependent variable of cortical thickness. These two variables may be capturing different biological bases of neuronal structure that are sensitive to dif- ferent between-subject factors in the two hemispheres.
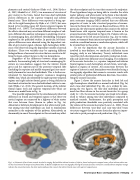
Regardless of the differences between image analysis methods, the mounting body of structural neuroimaging lit- erature on tone-deafness suggests that the inferior frontal gyrus and the superior part of the posterior temporal lobe (including the superior and middle temporal gyri) are consis- tently abnormal in tone-deaf individuals. This is further cor- roborated by functional magnetic resonance imagining (fMRI) data, which also identified the right superior temporal regions and right inferior frontal gyrus as being deficient in functional activation for tone-deaf individuals relative to con- trols (Hyde et al., 2010). The general locations of the inferior frontal region (red) and superior temporal lobe (blue) are shown on a model brain in Fig. 1a.
One possible explanation for the simultaneously observed differences in frontal and temporal regions is that these two cortical regions are connected by a highway of white matter that runs between them (shown in yellow in Fig. 1a). Abnormal or deficient development in this white matter path- way among tone-deaf individuals can lead to simultaneous anomalies in the endpoints of this pathway. Alternatively, an abnormality in one of the endpoints in this network may affect
the other endpoint and the tract that connects the endpoints. These hypotheses hinge on being able to visualize the white matter pathway, and white matter in the brain is best identifi- able using Diffusion Tensor Imaging (DTI), a structural mag- netic resonance imaging (MRI) method that uses diffusion properties of water to infer structural properties of connec- tions in the brain (for a review, see Basser and Jones, 2002). In particular, the white matter pathway that connects the inferior frontal areas with superior temporal areas is known as the arcuate fasciculus (illustrated in Figure 1b). Patients with iso- lated damage to the left arcuate fasciculus (e.g., due to stroke, tumor, or traumatic brain injury) are typically unable to repeat words in a condition known as conduction aphasia, which will be revisited later in this article.
To test the hypothesis that the arcuate fasciculus is involved in tone-deafness, we conducted a diffusion tensor imaging study in our laboratory. Twenty individuals were tested for pitch discrimination and pitch production thresh- olds and underwent diffusion tensor imaging. The endpoints of the arcuate fasciculus, i.e., superior temporal and inferior frontal regions in each hemisphere of each brain, were high- lighted as regions of interest. All connections between the regions of interest in each hemisphere were identified using a tractography algorithm that connects successive voxels with similar paths of preferential diffusion direction, thus identi- fying the arcuate fasciculus.
Figure 2 shows the arcuate fasciculus in both left and right hemispheres identified in a normal individual (Fig. 2a) and a tone-deaf individual (Fig. 2b). As is evident by com- paring the two figures, the tone-deaf individual possesses much less fiber volume in the arcuate fasciculus. In a group study (n = 20), the arcuate fasciculus was found to be dimin- ished in volume among tone-deaf individuals compared to matched controls. Furthermore, the pitch perception and pitch production thresholds were positively correlated with the volume of the arcuate fasciculus (Loui et al., 2009). These differences between tone-deaf and non-tone-deaf brain structures suggest that the arcuate fasciculus plays an impor- tant role in pitch perception and production. (Fig. 2).
Fig. 1. a) Schematic of basic brain network involved in singing. Red and blue indicate locations of inferior frontal and superior temporal areas; yellow indicates white mat- ter that runs between the temporal and frontal lobes. b) Results of diffusion tensor tractography showing the arcuate fasciculus overlaid on a standard brain template. Orange indicates the arcuate fasciculus, the white matter pathway that connects the inferior frontal areas with superior temporal areas.
Neurological Bases and Applications of Singing 29