Page 18 - Summer2022
P. 18
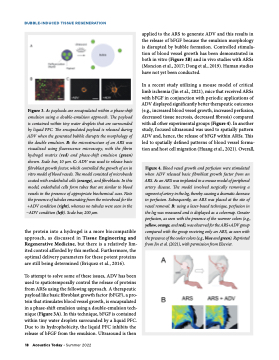
BUBBLE-INDUCED TISSUE REGENERATION applied to the ARS to generate ADV and this results in the release of bFGF because the emulsion morphology is disrupted by bubble formation. Controlled stimula- tion of blood vessel growth has been demonstrated in both in vitro (Figure 3B) and in vivo studies with ARSs (Moncion et al., 2017; Dong et al., 2019). Human studies have not yet been conducted. In a recent study utilizing a mouse model of critical limb ischemia (Jin et al., 2021), mice that received ARSs with bFGF in conjunction with periodic applications of ADV displayed significantly better therapeutic outcomes (e.g., increased blood vessel growth, increased perfusion, decreased tissue necrosis, decreased fibrosis) compared with all other experimental groups (Figure 4). In another study, focused ultrasound was used to spatially pattern ADV and, hence, the release of bFGF within ARSs. This led to spatially defined patterns of blood vessel forma- tion and host cell migration (Huang et al., 2021). Overall, Figure 3. A: payloads are encapsulated within a phase-shift emulsion using a double-emulsion approach. The payload is contained within tiny water droplets that are surrounded by liquid PFC. The encapsulated payload is released during ADV when the generated bubble disrupts the morphology of the double emulsion. B: the microstructure of an ARS was visualized using fluorescence microscopy, with the fibrin hydrogel matrix (red) and phase-shift emulsion (green) shown. Scale bar, 10 μm. C: ADV was used to release basic fibroblast growth factor, which controlled the growth of an in vitro model of blood vessels. The model consisted of microbeads coated with endothelial cells (orange), and fibroblasts. In this model, endothelial cells form tubes that are similar to blood vessels in the presence of appropriate biochemical cues. Note the presence of tubules emanating from the microbead for the +ADV condition (right), whereas no tubules were seen in the −ADV condition (left). Scale bar, 200 μm. Figure 4. Blood vessel growth and perfusion were stimulated when ADV released basic fibroblast growth factor from an ARS. A: an ARS was implanted in a mouse model of peripheral artery disease. The model involved surgically removing a segment of artery in the leg, thereby causing a dramatic decrease in perfusion. Subsequently, an ARS was placed at the site of vessel removal. B: using a laser-based technique, perfusion in the leg was measured and is displayed as a colormap. Greater perfusion, as seen with the presence of the warmer colors (e.g., yellow, orange, and red), was observed for the ARS+ADV group compared with the group receiving only an ARS, as seen with the presence of the cooler colors (e.g., blue and green). Reprinted from Jin et al. (2021), with permission from Elsevier. the protein into a hydrogel is a more biocompatible approach, as discussed in Tissue Engineering and Regenerative Medicine, but there is a relatively lim- ited control afforded by this method. Furthermore, the optimal delivery parameters for these potent proteins are still being determined (Briquez et al., 2016). To attempt to solve some of these issues, ADV has been used to spatiotemporally control the release of proteins from ARSs using the following approach. A therapeutic payload like basic fibroblast growth factor (bFGF), a pro- tein that stimulates blood vessel growth, is encapsulated in a phase-shift emulsion using a double-emulsion tech- nique (Figure 3A). In this technique, bFGF is contained within tiny water droplets surrounded by a liquid PFC. Due to its hydrophobicity, the liquid PFC inhibits the release of bFGF from the emulsion. Ultrasound is then 18 Acoustics Today • Summer 2022