Page 13 - Volume 8, Issue 4 - Winter 2012
P. 13
they utilized real-time MR thermome- try to confirm focal size and location during treatment, as well as monitor temperature increases.
As well as being able to generate
heat, ultrasound is capable of inducing
mechanical bioeffects within tissue.
Ultrasonic cavitation refers to either
stable cavitation, which is the periodic
oscillation of a microbubble under an
oscillating pressure field, or inertial
cavitation, which is the formation and
violent collapse of a cavity under very
high acoustic pressures. Inertial cavita-
tion is capable of causing tissue vapor-
14
events are occurring.
Stable cavitation is associated with sub-harmonic and ultra-harmon- ic acoustic emissions and inertia cavi- tation is associated with the emission of acoustic broadband noise. Passive cavi- tation detection has been adopted to measure the spectrum and intensity of an acoustic signal to assess whether any desirable or undesirable cavitation
15,16
ization.
Development of transcranial arrays
Modern transcranial transducer arrays take the form of a large hemi- sphere composed of a large number of high power transducer elements. Choice of frequency is an important consideration when designing a tran- scranial phased array. Ultrasound at lower frequencies suffers less attenua-
4
smaller elements to populate an entire array, to achieve adequate focusing and avoid grating lobes. It has been deter- mined that optimal transcranial focus- ing for thermal treatments occurs at
6,17
out the need for phase correction.
The first MR-guided clinical tran- scranial hemispherical phased array system was the Exablate 3000 devel- oped by Insightec (Haifa, Israel). It had a diameter of 30 cm and consisted of 512 elements operating at 670 kHz that were coupled to a 512-channel driving system capable of producing 800 W of
acoustic power.19 The system also implemented treatment planning, MR feedback control, and a water cooling system to reduce skull heating. The resulting half power focus size was 2 mmby4mm.
Dividing the hemispherical array into a larger number of smaller trans- ducer elements increases the ability of the phased array to steer and to correct
6
for skull distortion. However, increas-
ing the number of elements greatly increases the technical complexity, as it not only requires additional transducer elements, but also independent driving amplifiers and matching circuitry. The use of lateral mode transducer ele-
tion and distortion through the skull. Higher frequencies result in tighter focusing, higher pressure amplitudes, but also greater attenuation and focal distortion. Furthermore, element sizes required for steering are proportional to the wavelength, meaning that higher frequencies require a larger number of
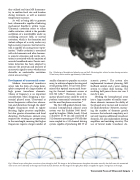
Fig. 1. Rendering of hemispherical phased array and skull, illustrating their relative locations during sonication. Virtual array shown contains approximately 1,000 elements.
However, lower fre- quency phased arrays could be used to perform transcranial treatments with-
18
600-700 kHz.
Fig. 2. Phased arrays utilize phase correction to focus through a skull. The image on the left demonstrates how phase aberration caused by the skull causes the transmitted waves to not arrive coherently at the target and results in focal distortion. For the image on the right, phase delays are applied to regain a strong focus at the target.
Transcranial Focused Ultrasound Surgery 9